-
Call for abstract: 4th International Conference Innovations in Cancer Research and Regenerative Medicine
Dear Colleagues, On behalf of the Organizing Committee, we are pleased to inform you that “The 4th international conference Innovations in Cancer Research and Regenerative Medicine (iCRRM)“, the international conference about Cancer Research and Regenerative Medicine co-organized by University of Science, VNU–HCM, Viet Nam; the University of Georgia, US; and Yonsei University, South Korea will…
-
Merry Christmas and Happy New Year 2019
With all my heart and soul, I would like to wish you a merry Christmas and a happy New Year
-
Emerging Sources Citation Index: Critical research and trends from emerging and less-established sources
The Emerging Sources Citation Index (ESCI) deepens and broadens your current Web of Science coverage— delivering the most comprehensive discovery across areas of research in journals of regional importance, not found in more established journals. 10-year backfile now available! Supporting regional discovery needs, ESCI connects to the more than 1.4 billion searchable citations across the…
-
Biomedical Research and Therapy receives new Index Copernicus Value (ICV) 2017
Biomedical Research and Therapy (BMRAT) receives the new Index Copernicus Value (ICV) from Index Copernicus International (ICI). The new ICV value is 100. This value significantly increases from 83.1 in 2016 to 100 in 2017. This result shows that the quality of Biomedical Research and Therapy significantly increases from 2016. For more information, please read…
-
Publons Release Inaugural Global State of Peer Review Report
Largest Ever Study of Peer ReviewShines Light on Under-Representation in the Peer Review Process and the Growing Demand for Non-Western Researchers to Participate LONDON, Sept. 7, 2018 /PRNewswire/ — Publons – the leading scholarly peer review platform, today launched the Global State of Peer Review report, the largest ever study of peer review. The report brings…
-
Journal Citation Reports: A Primer on the JCR and Journal Impact Factor
The Journal Citation Reports, first published in 1975, is an annual compendium of citation data that provides a systematic and objective means to assess influence and impact at the journal and category levels. It contains reports on citation performance for science and social science journals and the relationships between citing and cited journals. To receive…
-
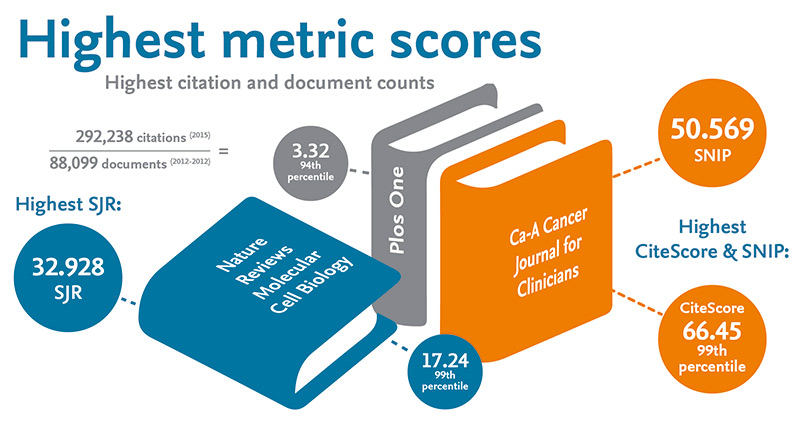
CiteScore 2017 values now available
Don’t speculate—validate. Let CiteScore™ metrics guide you to the right journals. The CiteScore 2017 values are here! Establishing a new standard for measuring serial citation impact, CiteScore™ metrics are freely available for more than 23,350 titles — 12,000 more titles than the leading competitor. Powered by Scopus, CiteScore metrics are a comprehensive, current, transparent and…
-
Two journals of Biomedpress are added to Scopus from April 2018
Today, the Scopus’s title list is updated to April 2018. In this list, there are 2 journals of Biomedpress, included Biomedical Research and Therapy (ISSN 21984093); and Progress in Stem Cell (ISSN 21994633). In order to promote scientific and technological publications about biomedicine, the Biomedpress, a publishing department of Stem Cell Institute, VNUHCM University of…
-
BioMedPress will publish Asian Journal of Health Sciences from 2018
Asian Journal of Health Sciences (2347-5218) has been published by Sydus Publishing (India) since 2013. This journal is a multidisciplinary, peer reviewed, open access, halfyearly journal which publishes a wide range of scientific works including original research papers, case reports, reviews, audits, editorials, book reviews. It includes work from basic science, clinical science, dental, nursing…
-
Call for Editorial Board Members, 2018-2020
On behalf of Editor-in-Chief Phuc Van Pham, we are pleased to announce that Biomedical Research and Therapy, Asian Journal of Health Sciences (AJHS) is seeking qualified candidates who are willing to serve, if elected, in the position of Editorial Board Member. According to the charter of BioMedPress, the Member term is for 3 years, with…