European regulators have recommended Alofisel, a new advanced therapy to treat complex perianal fistulas in patients with Crohn’s disease.
The treatment is now just a step away from being the first ever allogeneic stem cell therapy approved in Europe, and will put the Belgium-based biotech company into the vanguard of cell and gene therapy companies which have emerged in 2017. Allogeneic stem cell therapy allows “off the shelf” cells to be used in therapy, rather than having to take cells from each individual patient, allowing a relatively fast and inexpensive therapy. Alofisel represents a more modest improvement over existing treatment compared to some of the dazzling results seen in leukaemia and haemophilia, but nevertheless represents progress in a hard-to-treat condition.
The EMA’s CHMP committee recommendation is for Alofisel (darvadstrocel) to treat the serious complication, which the Tigenix and Takeda say affects around 28% of patients with Crohn’s disease in the first 20 years of living with the disease. Once given final approval by the European Commission, Takeda will market the drug in the EU following an agreement with Tigenix signed last year.
In the likely event of an EU licence for the therapy, Takeda will pay a 15 million euro fee to Tigenix. Apart from affecting the lining of the bowel, Crohn’s disease can cause inflammation deeper into the bowel wall, causing an abnormal passageway to open between the bowel and the outside of the body.
This can lead to incontinence and sepsis, and complex fistulas are more difficult than simple fistulas. Alofisel (darvadstrocel) is based on expanded adipose stem cells which, once activated, impair proliferation of lymphocytes and reduce the release of pro-inflammatory cytokines at inflammation sites. This immunoregulatory activity reduces inflammation and may allow the tissues around the fistula tract to heal.
Dr. María Pascual, vp regulatory affairs and corporate quality at TiGenix, said, “We believe that this first approval recommendation for an allogeneic stem cell therapy in Europe reflects the maturity of our technology and its potential to offer new approaches for difficult to treat conditions.” Presuming that EU approval can be secured, the next major milestone will be FDA approval. In March this year the company received feedback from the FDA about its trial design, which should allow filing of the drug in 2018.
“We have worked closely with the EMA and provided a robust data package from a well-designed clinical trial with challenging endpoints,” she adds. “In parallel, we will continue working hard to obtain regulatory approval in the US and to develop Cx601 for additional indications, to fulfil our aim of allowing patients to benefit from the full potential of Cx601 across multiple geographies and diseases.”
The CHMP’s recommendation is based phase 3 clinical trial involving 212 patients, which showed significant but not overwhelming superiority of the treatment compared to placebo. After 24 weeks of treatment, half of the patients treated with Alofisel (49.5%) were in remission, compared to a third of the patients under placebo. An extended ongoing follow-up study, which will cover a period of up to 104 weeks of treatment, has supported this result to date.
Although there is a moderate difference between the treatment groups, the effect is considered to be clinically meaningful when other treatment options for fistulas have failed. The most common side effects observed include anal abscess and fistula, as well as procedural pain and proctalgia. The CHMP backed an initial decision by the EMA’s Committee for Advanced Therapies, a special committee for advanced gene or cell therapies.
TiGenix has a second adipose-derived product, Cx611, is undergoing a phase 1/2 trial in severe sepsis, a deadly condition which affects millions of patients across the world ever year. It also has AlloCSC-01, which targets acute ischemic heart disease, which has so far produced positive results in a Phase 1/2 trial in acute myocardial infarction.
MEDICAL NEED
Crohn’s disease is a chronic inflammatory disease of the intestine. A complication of the disease is the development of complex perianal fistulas. A perianal fistula is an abnormal connection between the perianal space and the outside skin surface which causes a significant negative impact on quality of life. A fistula is considered to be complex when its treatment involves a high risk of causing anal incontinence, the fistulous tract crosses more than 30% of the external sphincter, several tracts are found, it is recurrent, or when the patient has incontinence, local irritation, or Crohn’s disease.
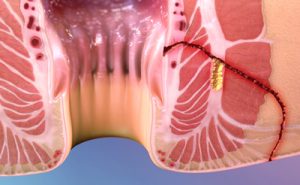
Complex perianal fistulas in patients suffering from Crohn’s disease tend to occur in individuals between the ages of 20 and 40, though 10-15% of patients are diagnosed before adulthood.
Current treatment options for complex fistulas in patients with Crohn’s disease include the use of antibiotics, immunosuppressants, anti TNFs and surgery. However, these treatment options are marked by poor long term efficacy and considerable safety issues: treatment with anti TNFs is associated with low remission and high relapse as well as with safety concerns when they are used long term. In the case of surgery, the treatment is associated with a high risk of recurrence and of complications which can include incontinence, non-healing wounds and abscesses.
Cx601
Cx601 is a local administration of expanded adipose-derived stem cells (eASCs) for the treatment of complex perianal fistulas in Crohn’s disease patients. The treatment is administered as a single dose and has been proven to have long-term efficacy in the healing of complex perianal fistulas in Crohn’s disease patients (ADMIRE-CD study completed in 2015 with positive 2 year follow-up data). The 24-week results of this trial were published in The Lancet in July 2016.
Cx601 has been designated as an orphan drug by the EMA and SwissMedic, in Switzerland.
On 4th July 2016, Takeda Pharmaceuticals acquired an exclusive right to develop and commercialize Cx601 for complex perianal fistulas in Crohn’s disease patients outside of the U.S. Takeda is a leading pharmaceutical company in the gastroenterology space. TiGenix retains full rights to the product in the US as well as to the development of Cx601 in other indications.
(Sources: https://pharmaphorum.com/news/tigenixtakeda-stem-cell-therapy-backed-eu-regulators/; http://tigenix.com/product-candidates/cx601/)
Leave a Reply